CBS News Live
CBS News Baltimore: Local News, Weather & More
Watch CBS News

Once complete, Amtrak's Frederick Douglass Tunnel, a multi-billion dollar project, will connect commuters across the state faster than ever.

Dozens of Catholic churches in Baltimore may be forced to close their doors in the Archdiocese's "Seek the City to Come" initiative.

Clouds increase late tonight with showers returning Wednesday. Weekend weather is trending dry and nice.

Multiple teenagers were arrested in and around Towson Town Center after a series of incidents over the weekend, according to Baltimore County Police.

The late Dr. Rudiger Breitenecker began this work at Greater Baltimore Medical Center before forensic science became fundamental to the investigative process.

A fourth body was recovered Sunday at the site of the Key Bridge collapse, according to the Unified Command.

People will hit the pavement this weekend to participate in Kennedy Krieger Institute's "Roar for Kids 5K Run" fundraiser which will help support research and programs.

A man is wanted for a burglary and vandalism at Christ the King Catholic Church in Glen Burnie, police said.

A 5-year-old girl is recovering after being attacked by two dogs in Anne Arundel County, police told WJZ.

Derek Beasley has your Tuesday night forecast (4/16/2024)

Cranes continue to move a significant portion of the Key Bridge out of the Patapsco River.

The bridge crumbled nearly three weeks ago after its support column was struck by a malfunctioning cargo ship in the early morning hours of March 26

A fourth body was recovered Sunday at the site of the Key Bridge collapse, according to the Unified Command.

Orioles pitcher Grayson Rodriguez allowed two runs on four base hits in six innings.

BARCS Animal Shelter in Baltimore City brought their adorable pups, including "Analu" and "Liko" to play on the field with some of the Baltimore Orioles.

Ryan O'Hearn, Cedric Mullins and Gunnar Henderson each went yard in the Orioles' 7-4 victory over the Twins.

Baltimore native Angel Reese was selected seventh overall by the Chicago Sky in the 2024 WNBA Draft.

April 15 is widely known as "Jackie Robinson Day" across Major League Baseball.
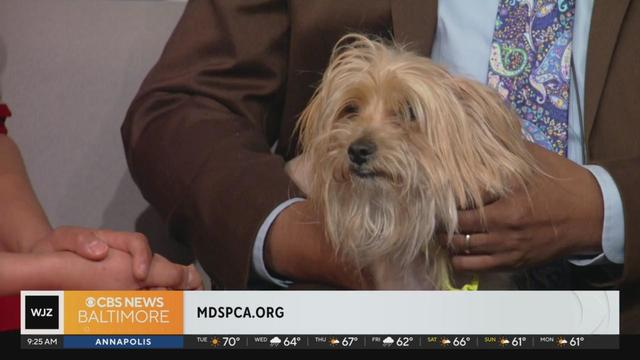
Champ is a Yorkshire Terrier found as a stray dog in Towson, Maryland

The steakhouse is located at MGM National Harbor Resort and Casino

The Maryland Chapter of the Cystic Fibrosis Foundation will host the 8th Annual "Feastival, A Food Adventure for a Cure" on April 13, 2024 from 1 to 4 pm at the Inner Harbor’s West Shore Park.

Carla currently hosts the MAX series "Chasing Flavor," where she travels the world to trace the history and lineage of some of our favorite foods.
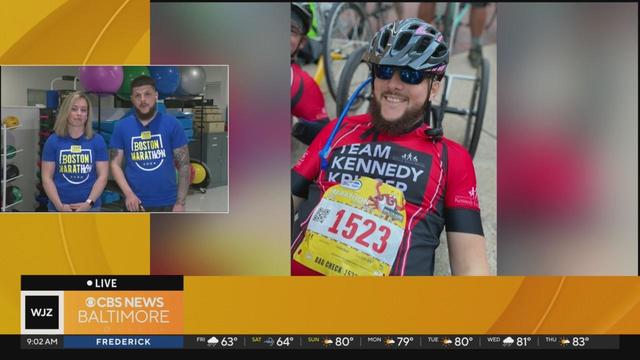
Kennedy Krieger Institute is supporting two local handcyclists representing its adaptive sports programs in the Boston Marathon.
Dr. Viola joins us in studio to discuss why parents need to focus on skin care for young children and the risks of high sun exposure.
Nancy Johnston, founder and CEO of Truuce, joins us in studio to share how her duvet covers and bedding line ensure sheets stay on and tucked.

Baltimore Center Stage is preparing for the regional premiere production of the Pulitzer Prize-winning comedy-drama The Hot Wing King,
The University of Maryland, Baltimore County agreed to a long list of changes prescribed by the U.S. Department of Justice, but experts say true reform requires a culture change, the Baltimore Banner's Kristen Griffith reports.

Derek Beasley has your Tuesday night forecast (4/16/2024)

Appeals Court rejects GTTF officer's early release request

Slides from cold case sexual assaults preserved, survivors asked to come forward

Baltimore residents concerned by construction of major rail project
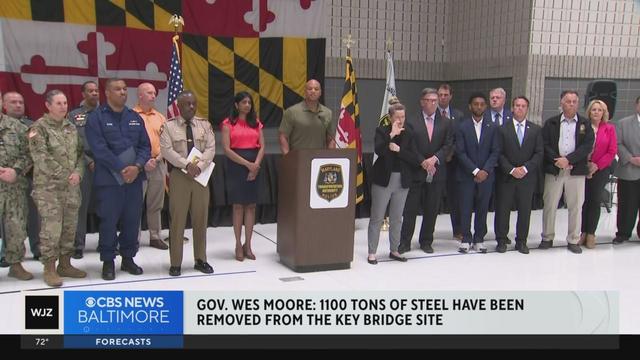
Heavy debris removed from Key Bridge collapse site, and more top stories

Once complete, Amtrak's Frederick Douglass Tunnel, a multi-billion dollar project, will connect commuters across the state faster than ever.

Dozens of Catholic churches in Baltimore may be forced to close their doors in the Archdiocese's "Seek the City to Come" initiative.

Clouds increase late tonight with showers returning Wednesday. Weekend weather is trending dry and nice.

Multiple teenagers were arrested in and around Towson Town Center after a series of incidents over the weekend, according to Baltimore County Police.

The late Dr. Rudiger Breitenecker began this work at Greater Baltimore Medical Center before forensic science became fundamental to the investigative process.

Maryland is the host state for National Work Zone Safety Week this year, so state leaders gathered Tuesday to remind drivers to be safer around work zones.

The bridge crumbled nearly three weeks ago after its support column was struck by a malfunctioning cargo ship in the early morning hours of March 26

This year, trillions of cicadas will appear in several U.S. states, including in parts of Maryland.

Police are investigating the deaths of three people in Aberdeen as homicides after a fire led emergency responders to the scene Monday morning.

Perhaps fueled by NCAA March Madness betting, the state's 13 retail and 12 mobile sportsbooks handled $536 million in wagers last month.

Champ is a Yorkshire Terrier found as a stray dog in Towson, Maryland

The steakhouse is located at MGM National Harbor Resort and Casino

The Maryland Chapter of the Cystic Fibrosis Foundation will host the 8th Annual "Feastival, A Food Adventure for a Cure" on April 13, 2024 from 1 to 4 pm at the Inner Harbor’s West Shore Park.

Carla currently hosts the MAX series "Chasing Flavor," where she travels the world to trace the history and lineage of some of our favorite foods.

Kennedy Krieger Institute is supporting two local handcyclists representing its adaptive sports programs in the Boston Marathon.

Orioles pitcher Grayson Rodriguez allowed two runs on four base hits in six innings.

BARCS Animal Shelter in Baltimore City brought their adorable pups, including "Analu" and "Liko" to play on the field with some of the Baltimore Orioles.

Ryan O'Hearn, Cedric Mullins and Gunnar Henderson each went yard in the Orioles' 7-4 victory over the Twins.

Baltimore native Angel Reese was selected seventh overall by the Chicago Sky in the 2024 WNBA Draft.

April 15 is widely known as "Jackie Robinson Day" across Major League Baseball.

The Supreme Court seemed divided over a case challenging the scope of a federal obstruction law that prosecutors have used to charge more than 300 Jan. 6 defendants.

The investigation is being conducted by Maryland's U.S. Attorney's Office and the FBI.

The White House is turning to other methods to erase student debt after the Supreme Court blocked its broader effort to forgive loans.

Baltimore voters broadly approve of elected leaders' response to the deadly collapse of the Francis Scott Key Bridge last month, according to a newly released poll.

The two-page bill, dubbed the Baltimore BRIDGE Relief Act, would have the federal government cover 100% of the cost of replacing the bridge.

When it comes to vintage style, art and innovation—Baltimore is home to it all.

The University of Maryland Medical Center was granted the American Nurses Credentialing Center's (ANCC) Magnet for the fourth time in a row.

Magali Uroza comes from a Mexican immigrant family and remembers the challenges of learning English while growing up in Baltimore.

It is the cherry trees' second-earliest peak bloom on record and follows one of Washington's warmest recorded winters. In 1990, the trees blossomed on March 15.
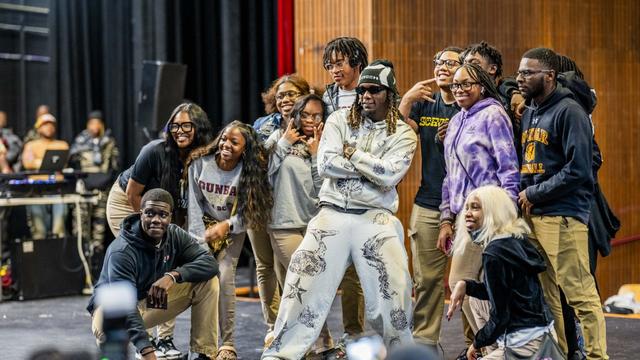
Georgia rapper Offset surprised students at Dunbar High School on Tuesday for their stellar academic performance and attendance according to the Baltimore Police Department.

The Maryland Food Bank is on pace to distribute more than 52 million pounds of food, which is a 20 percent jump in comparison to pre-COVID times.

It has long been a popular resource in the community, easily accessible to many college students and neighbors.

Has mom and dad's bank been open too long at your house?

According to a report from the National Institute on Retirement Security, 40% of Gen Xers have zero dollars saved for retirement.

According to Bankrate, Baltimore is one of the top 10 most affordable cities for buying used cars.

Primanti Bros. on Tuesday announced plans to expand to the Baltimore region this spring.

CVS is closing dozens of pharmacies inside Target stores in 2024 as the store and other retail pharmacy chains face increasing difficulties.

The wildly popular chicken finger joint opened its doors Tuesday at the Snowden River Shopping Center.

The council passed the "Bring Your Own Bag Act" with a bipartisan vote in February.

Baltimore is one of 31 designees announced Monday, picked from nearly 400 applicants.

Most worrisome gaps involve cancer chemotherapy drugs, ER medications and and therapies for ADHD.

The prepackaged boxes of deli meat, cheese and crackers are not a healthy choice for kids, advocacy group says.

The University of Maryland Medical Center was granted the American Nurses Credentialing Center's (ANCC) Magnet for the fourth time in a row.

The state aims to force the company to make necessary repairs and impose fines of up to $25,000 per violation.
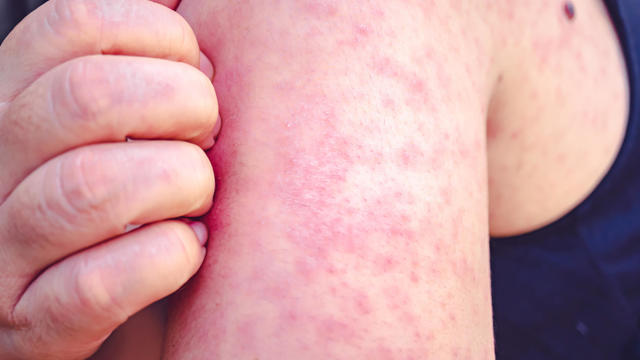
Infections of the highly contagious measles virus have been reported in 17 states in the first three months of 2024.

In the 1,000th episode, titled "A Thousand Yards," NCIS comes under attack by a mysterious enemy from the past.

A Billy Joel special on CBS and Paramount+ will air again after it was cut off in the middle of the singer's performance of "Piano Man."

The show, renowned for its thrilling investigations and dedicated team, has become a cornerstone of television drama.

O.J. Simpson was famously acquitted in the 1994 slayings of his ex-wife, Nicole Brown Simpson, and her friend Ronald Goldman.

Most of Maryland will get a partial eclipse on that blocks 85-90% of the sun, according to NASA.

Clouds increase late tonight with showers returning Wednesday. Weekend weather is trending dry and nice.

Derek Beasley has your Tuesday night forecast (4/16/2024)

Derek Beasley has your Tuesday evening forecast (4/16/2024)

Derek Beasley has your Tuesday evening forecast (4/16/2024)

We are waking up to refreshing air in the wake of last night's cold front. As cooler, drier air settles across the state, morning temperatures are falling into the 50s.

Derek Beasley has your Tuesday night forecast (4/16/2024)

Appeals Court rejects GTTF officer's early release request

Slides from cold case sexual assaults preserved, survivors asked to come forward

Baltimore residents concerned by construction of major rail project

Heavy debris removed from Key Bridge collapse site, and more top stories