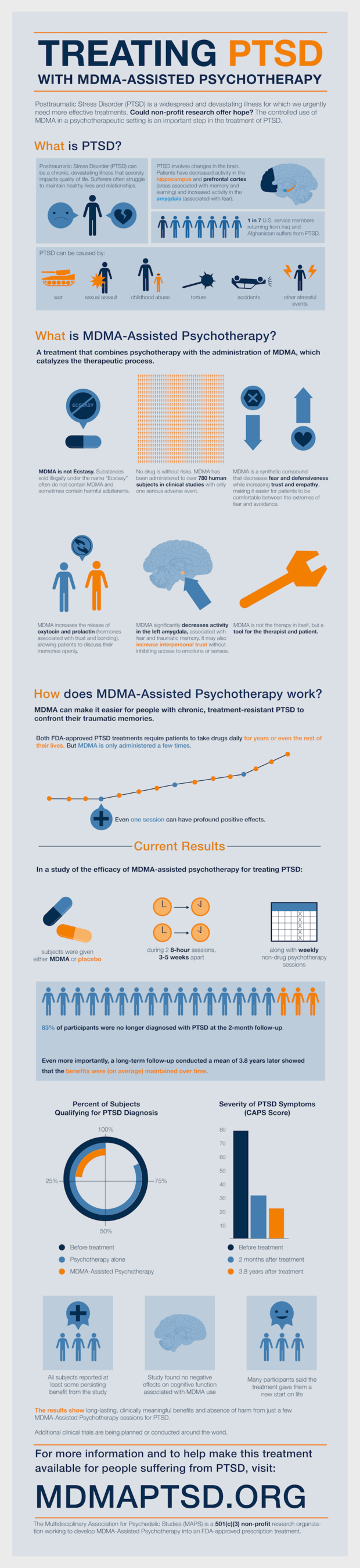
FDA Approves Expanded Research Into MDMA-Assisted Psychotherapy For PTSD Patients
SANTA CRUZ, CA (WJZ) -- The U.S. Food and Drug Administration recently approved an expansion of a trial that allows researchers to see how MDMA could help people with posttraumatic stress disorder.
The nonprofit, Multidisciplinary Association for Psychedelic Studies or MAPS, applied for the expansion last year and the FDA approved it on Dec. 20, 2019. The Expanded Access program that would allow 50 PTSD patients to receive MDMA-assisted psychotherapy.
This program will be limited to patients with moderate to severe treatment-resistant PTSD. The patients will have to cover the costs on their own.
MDMA, or 3,4-methylenedioxy-methamphetamine, is a synthetic drug that alters mood and perception, according to NIH. According to MAPS, MDMA is different from ecstasy or "molly" that are sold as a street or club drug that mixed with unknown or dangerous substances.
"In laboratory studies, pure MDMA has been proven sufficiently safe for human consumption when taken a limited number of times in moderate doses," MAPS said on its website.
READ MORE:
- More Than A Trip: Psychedelic Drugs Being Used To Help People Quit Smoking In Just One Dose
- Psychedelics: Can Getting High Improve Your Mental Health?
- MDMA, the main ingredient in ecstasy, could be key in helping veterans with PTSD
"We commend FDA for recognizing the great unmet medical need of PTSD by allowing access to MDMA-assisted psychotherapy on a compassionate basis for people with treatment-resistant PTSD," said MAPS Founder and Executive Director Rick Doblin, Ph.D. "We are delighted to begin generating real-world evidence about this potential new treatment."
Up to 10 qualifying treatment sites across the U.S. will be selected to participate in the program over the next few months.
MAPS is currently running Phase 3 clinical trials of MDMA-assisted psychotherapy for PTSD at 15 sites in the U.S., Canada, and Israel.
The FDA granted Breakthrough Therapy Designation to MDMA-assisted psychotherapy for PTSD in August 2017.
"It starts by reducing activity in the amygdala, which is the fear-processing part of the brain, so that people's fearful emotions linked to trauma can be more easily recalled and processed," Doblin told CBS Evening News in 2018.
Once the drug produces feelings of safety, veterans can then access memories which had been crippling before. A study including 24 veterans showed PTSD was eliminated in 68 percent of vets treated with MDMA-assisted therapy and significantly reduced in the other 32 percent, CBS reported.
The Phase 3 trials are expected to be completed in 2021, meaning that the FDA could approve the treatment as early as 2022. MAPS is also initiating Phase 2 trials in Europe, starting in January.
Learn more about MAPS' research here. Click here if you'd like to participate in a trial.