CBS News Live
CBS News Baltimore: Local News, Weather & More
Watch CBS News

Ships stuck in the Port of Baltimore since the collapse of the Francis Scott Key Bridge last month are finally departing Thursday.

Follow live updates of Donald Trump's New York criminal trial, where former National Enquirer boss David Pecker is testifying for the third day.

Pikesville High School's principal Eric Eiswert was implicated in an audio recording making rounds on social media containing racist and antisemitic comments, but police say the voice was AI-generated.

On Wednesday in Baltimore City, people gathered at Johns Hopkins University to stand in solidary with Gaza and students at other college campuses to call for disinvestment from Israel.
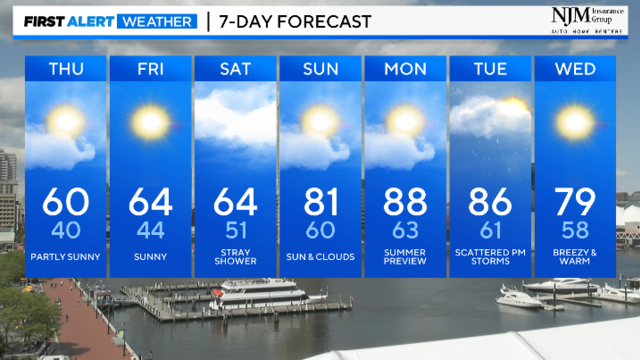
Expect a chilly breeze with temperatures falling into the 40s tonight. Thursday's highs stay in the 50s to near 60.

Rapper Busta Rhymes and funk rockers Morris Day and The Time are headlining the 47th annual AFRAM this year in Baltimore.

The Supreme Court convened to consider whether former President Donald Trump is entitled to broad immunity from criminal charges in the 2020 election case.

Wright has served as the Interim State Superintendent of Schools since October 23, 2023.

Airlines must give automatic cash refunds to all canceled and significantly delayed flights, according to new Department of Transportation rules
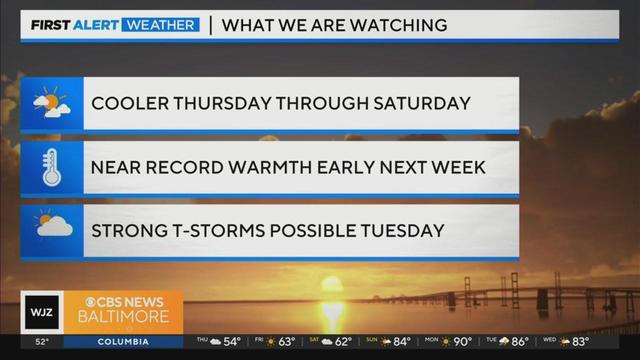
Meteorologist Meg McNamara has your Thursday morning forecast 4/25/2024

Ships stuck in the Port of Baltimore since the collapse of the Francis Scott Key Bridge last month are finally departing Thursday.

A fourth temporary channel is scheduled to open at the Port of Baltimore on Thursday, according to the U.S. Coast Guard Captain of the Port.

Since the Key Bridge collapse more than four weeks ago, the Port of Baltimore's main channel has been closed.

The three-day NFL Draft starts on Thursday, and several local draft hopefuls could have their lifelong dreams come true.

The Baltimore Ravens will attempt to bolster their roster this week with new rookies from the NFL Draft.

The Baltimore Ravens will look to add to their already talented roster through this week's NFL Draft.

Henderson homered, doubled, singled and was hit by a pitch while scoring three runs.

Bateman, drafted 27th overall, became the first first-round wide receiver to sign a second contract with the Ravens.
UMD is celebrating Maryland Day with a slew of events

MPT airs 30 programs through Chesapeake Bay Week a

Jim Henson was born and raised in Mississippi but moved with his family to Maryland when he was 10 years old. He went to the University of Maryland where he met his wife and partner, Jane, in a puppetry class.

The conference delivers opportunities for aspiring enterprisers to connect with seasoned entrepreneurs, interact with exhibitors, and attend education sessions.

Flowers by Chris joins WJZ to talk about business and tips on planting and gardening this spring

Where's Marty? Learning about X-Laser in Howard County.

The event features live performances from local gospel artists

The home on Crafton Road near Avenal Road caught on fire around 11 p.m. and exploded, shaking a neighborhood awake.

The program operates through a series of anywhere from 4-6 workshops that each focus on a particular aspect of learning and development, and how parenting practices that incorporate purposeful play can support each particular domain.

Today we are live with local meteorite expert Mike Hankey, who is showing us his Meteorite collection and talking to us about how he can track down meteorites.

UMD is celebrating Maryland Day with a slew of events
With Hampden's HonFest permanently wrapped, small business owners are planning a one-day festival called Hampden Highlights for June

MPT airs 30 programs through Chesapeake Bay Week a
Mike Hankey of Monkton is a software developer, entrepreneur, amateur astronomer, astrophotographer, meteor observer and meteorite hunter.

Ships stuck in the Port of Baltimore since the collapse of the Francis Scott Key Bridge last month are finally departing Thursday.

Follow live updates of Donald Trump's New York criminal trial, where former National Enquirer boss David Pecker is testifying for the third day.

Pikesville High School's principal Eric Eiswert was implicated in an audio recording making rounds on social media containing racist and antisemitic comments, but police say the voice was AI-generated.

On Wednesday in Baltimore City, people gathered at Johns Hopkins University to stand in solidary with Gaza and students at other college campuses to call for disinvestment from Israel.

Expect a chilly breeze with temperatures falling into the 40s tonight. Thursday's highs stay in the 50s to near 60.

Ships stuck in the Port of Baltimore since the collapse of the Francis Scott Key Bridge last month are finally departing Thursday.

Rapper Busta Rhymes and funk rockers Morris Day and The Time are headlining the 47th annual AFRAM this year in Baltimore.

Pikesville High School's principal Eric Eiswert was implicated in an audio recording making rounds on social media containing racist and antisemitic comments, but police say the voice was AI-generated.

With foreign imports and the company's need for more money to get going, their plan to bring back manufacturing in Sparrows Point remains stalled.

The new charge accuses Clendaniel of illegally possessing a 12-gauge shotgun despite her spending more than a year in prison.

UMD is celebrating Maryland Day with a slew of events

MPT airs 30 programs through Chesapeake Bay Week a

Jim Henson was born and raised in Mississippi but moved with his family to Maryland when he was 10 years old. He went to the University of Maryland where he met his wife and partner, Jane, in a puppetry class.

The conference delivers opportunities for aspiring enterprisers to connect with seasoned entrepreneurs, interact with exhibitors, and attend education sessions.

Flowers by Chris joins WJZ to talk about business and tips on planting and gardening this spring

The three-day NFL Draft starts on Thursday, and several local draft hopefuls could have their lifelong dreams come true.

The Baltimore Ravens will attempt to bolster their roster this week with new rookies from the NFL Draft.

The Baltimore Ravens will look to add to their already talented roster through this week's NFL Draft.

Henderson homered, doubled, singled and was hit by a pitch while scoring three runs.

Bateman, drafted 27th overall, became the first first-round wide receiver to sign a second contract with the Ravens.

Follow live updates of Donald Trump's New York criminal trial, where former National Enquirer boss David Pecker is testifying for the third day.

The Supreme Court convened to consider whether former President Donald Trump is entitled to broad immunity from criminal charges in the 2020 election case.

Jurors in former President Donald Trump's trial in New York heard testimony from a former media executive about his efforts to bury negative stories about Trump before the 2016 presidential election.

Jurors in former President Donald Trump's criminal trial in New York got their first glimpse of the arguments both sides plan to make.

The Supreme Court will hear arguments Monday on whether bans on public camping constitute "cruel and unusual punishment" barred by the Eighth Amendment.

When it comes to vintage style, art and innovation—Baltimore is home to it all.

The University of Maryland Medical Center was granted the American Nurses Credentialing Center's (ANCC) Magnet for the fourth time in a row.

Magali Uroza comes from a Mexican immigrant family and remembers the challenges of learning English while growing up in Baltimore.

It is the cherry trees' second-earliest peak bloom on record and follows one of Washington's warmest recorded winters. In 1990, the trees blossomed on March 15.
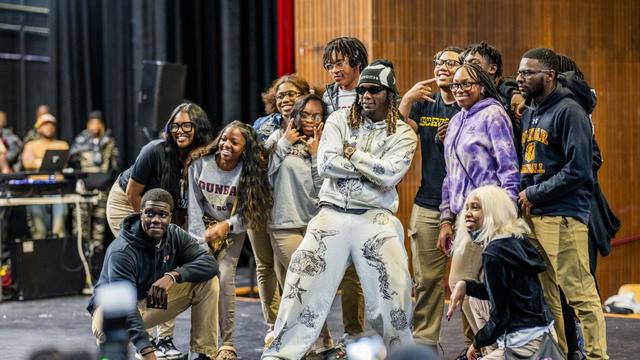
Georgia rapper Offset surprised students at Dunbar High School on Tuesday for their stellar academic performance and attendance according to the Baltimore Police Department.

The Maryland Food Bank is on pace to distribute more than 52 million pounds of food, which is a 20 percent jump in comparison to pre-COVID times.

It has long been a popular resource in the community, easily accessible to many college students and neighbors.

Has mom and dad's bank been open too long at your house?

According to a report from the National Institute on Retirement Security, 40% of Gen Xers have zero dollars saved for retirement.

According to Bankrate, Baltimore is one of the top 10 most affordable cities for buying used cars.

Primanti Bros. on Tuesday announced plans to expand to the Baltimore region this spring.

CVS is closing dozens of pharmacies inside Target stores in 2024 as the store and other retail pharmacy chains face increasing difficulties.

The wildly popular chicken finger joint opened its doors Tuesday at the Snowden River Shopping Center.

The council passed the "Bring Your Own Bag Act" with a bipartisan vote in February.

Baltimore is one of 31 designees announced Monday, picked from nearly 400 applicants.
Regular screenings are critical to identifying and treating cancer early. It's also important to be aware of any genetic mutations that already exist in your body.

Health officials are warning consumers not to consume Infinite Herbs basil sold at some Trader Joe's and Dierberg's stores after 12 people were sickened.

Most worrisome gaps involve cancer chemotherapy drugs, ER medications and and therapies for ADHD.

The prepackaged boxes of deli meat, cheese and crackers are not a healthy choice for kids, advocacy group says.

The University of Maryland Medical Center was granted the American Nurses Credentialing Center's (ANCC) Magnet for the fourth time in a row.

Rapper Busta Rhymes and funk rockers Morris Day and The Time are headlining the 47th annual AFRAM this year in Baltimore.

The significance of the song was amplified by praise from music legend Paul McCartney, who called the recording "magnificent" and appreciated its reinforcement of the civil rights message he intended when writing "Blackbird."

Charli XCX and Troye Sivan are stopping in Baltimore this fall on a joint tour.

In the 1,000th episode, titled "A Thousand Yards," NCIS comes under attack by a mysterious enemy from the past.

A Billy Joel special on CBS and Paramount+ will air again after it was cut off in the middle of the singer's performance of "Piano Man."

Meteorologist Meg McNamara has your Thursday morning forecast 4/25/2024
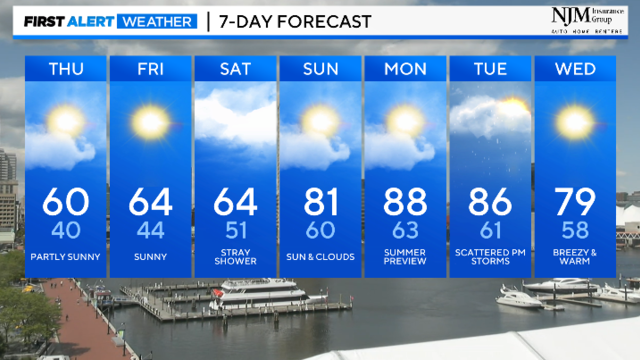
Temperatures will struggle to get to 60° today with summer like heat on the way.

Derek Beasley has your Wednesday night forecast (4/24/2024)

Derek Beasley has your Wednesday evening forecast (4/24/2024)

Derek Beasley has your Wednesday evening forecast (4/24/2024)

Today we are live with local meteorite expert Mike Hankey, who is showing us his Meteorite collection and talking to us about how he can track down meteorites.

UMD is celebrating Maryland Day with a slew of events

With Hampden's HonFest permanently wrapped, small business owners are planning a one-day festival called Hampden Highlights for June

MPT airs 30 programs through Chesapeake Bay Week a

Mike Hankey of Monkton is a software developer, entrepreneur, amateur astronomer, astrophotographer, meteor observer and meteorite hunter.