CBS News Live
CBS News Baltimore: Local News, Weather & More
Watch CBS News

Key Bridge salvage crews Thursday are pre-rigging a large portion of steel from the collapsed bridge before it is moved to nearby Sparrows Point.

Baltimore Ravens wide receiver Zay Flowers was cleared by the NFL after an investigation into violation of the personal conduct policy.

A Montgomery County high school student is facing charges after allegedly planning to commit a school shooting, according to police.

WJZ is partnering with The Baltimore Banner and WYPR Radio to host a debate on Tuesday, April 30 with the four leading Democratic mayoral candidates.
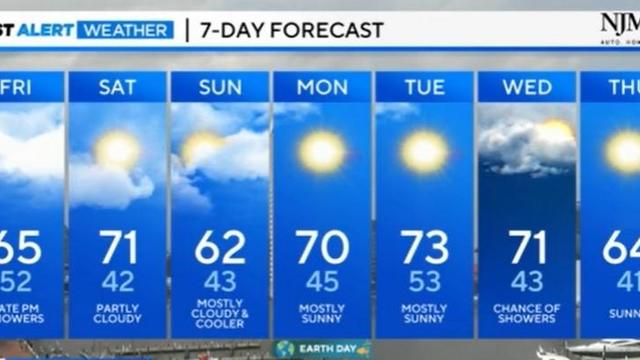
Rain chances to close out the week later Friday...followed by a dry weekend.

As salvage operations continue at the Francis Scott Key Bridge collapse site, the owners of the Dali have invoked a centuries-old maritime law requiring cargo owners to contribute to the costs of salvaging the ship and its cargo.

According to the Baltimore County Fire Department, the crash happened between Exits 18 and 19 in the Windsor Mill area.
The Baltimore music community will come together to support the families of the Key Bridge collapse victims on Thursday night.

For almost 100 years, Prigel Family Farm has continued to sustain and transform their agricultural heritage.

Derek Beasley has your Thursday evening forecast (4/18/2024)

Key Bridge salvage crews Thursday are pre-rigging a large portion of steel from the collapsed bridge before it is moved to nearby Sparrows Point.

As salvage operations continue at the Francis Scott Key Bridge collapse site, the owners of the Dali have invoked a centuries-old maritime law requiring cargo owners to contribute to the costs of salvaging the ship and its cargo.

About five miles, as the crow flies from what was once the Francis Scott Key Bridge, every decision in the complicated salvage operation is made at the command post.

Baltimore Ravens wide receiver Zay Flowers was cleared by the NFL after an investigation into violation of the personal conduct policy.

Former Baltimore Ravens' second-round draft pick J.K. Dobbins is reportedly signing with the Los Angeles Chargers.

The Orioles (12-6), winners of four in a row, defeated the Twins, 4-2.

Orioles pitcher Grayson Rodriguez allowed two runs on four base hits in six innings.

BARCS Animal Shelter in Baltimore City brought their adorable pups, including "Analu" and "Liko" to play on the field with some of the Baltimore Orioles.
Falsettos is about neurotic New Yorker Marvin, who leaves his wife Trina & son Jason for his lover Whizzer
Renowned Gospel artist Donald Lawrence is set to take that 2024 GospelFest at the Joseph Meyerhoff Symphony Hall, led by conductor Dr. Henry Panion III on April 27

City Ranch says its lessons are designed to be educational and engaging for people from all walks of life.
All of the proceeds from wellness day at Four Seasons will benefit Camp B'more, a weeklong urban summer camp

The event supports the medical center's sexual assault and domestic violence program, which provides confidential services to victims of all ages, at no cost to the patient.
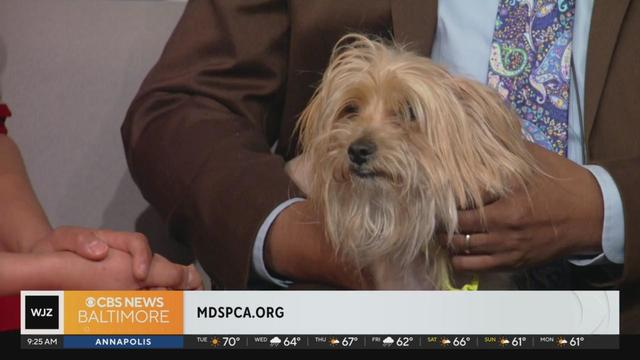
Champ is a Yorkshire Terrier found as a stray dog in Towson, Maryland

The steakhouse is located at MGM National Harbor Resort and Casino

The Maryland Chapter of the Cystic Fibrosis Foundation will host the 8th Annual "Feastival, A Food Adventure for a Cure" on April 13, 2024 from 1 to 4 pm at the Inner Harbor’s West Shore Park.

Carla currently hosts the MAX series "Chasing Flavor," where she travels the world to trace the history and lineage of some of our favorite foods.

Brigance Brigade Foundation set to host annual run to raise money for ALS

Army Corps gives close-up view of Key Bridge collapse site, plus more top news

Derek Beasley has your Thursday evening forecast (4/18/2024)

WJZ, Baltimore Banner, WYPR Radio will host Democratic mayoral debate on April 30

Prigel Family Farm works to expand agricultural education; offers summer camp for children

Key Bridge salvage crews Thursday are pre-rigging a large portion of steel from the collapsed bridge before it is moved to nearby Sparrows Point.

Baltimore Ravens wide receiver Zay Flowers was cleared by the NFL after an investigation into violation of the personal conduct policy.

A Montgomery County high school student is facing charges after allegedly planning to commit a school shooting, according to police.

WJZ is partnering with The Baltimore Banner and WYPR Radio to host a debate on Tuesday, April 30 with the four leading Democratic mayoral candidates.

Rain chances to close out the week later Friday...followed by a dry weekend.

It is certainly a seller's market right now, but if you're looking to buy a home in Maryland you won't want to wait around too long.

Kenneth Davis, 32, allegedly shot and killed 25-year-old Tracey Elizabeth Carrington as she was leaving a Baltimore bar.

Charli XCX and Troye Sivan are stopping in Baltimore this fall on a joint tour.

Baltimore prosecutors will reportedly announce an arrest Wednesday afternoon in the 2017 killing of an off-duty Washington DC police officer.

Hogan hopes to become the first Republican in more than 40 years to win a Senate seat in deep-blue Maryland, where Democrats outnumber Republicans 2-1 statewide.
Falsettos is about neurotic New Yorker Marvin, who leaves his wife Trina & son Jason for his lover Whizzer

Renowned Gospel artist Donald Lawrence is set to take that 2024 GospelFest at the Joseph Meyerhoff Symphony Hall, led by conductor Dr. Henry Panion III on April 27

City Ranch says its lessons are designed to be educational and engaging for people from all walks of life.

All of the proceeds from wellness day at Four Seasons will benefit Camp B'more, a weeklong urban summer camp

The event supports the medical center's sexual assault and domestic violence program, which provides confidential services to victims of all ages, at no cost to the patient.

Baltimore Ravens wide receiver Zay Flowers was cleared by the NFL after an investigation into violation of the personal conduct policy.

Former Baltimore Ravens' second-round draft pick J.K. Dobbins is reportedly signing with the Los Angeles Chargers.

The Orioles (12-6), winners of four in a row, defeated the Twins, 4-2.

Orioles pitcher Grayson Rodriguez allowed two runs on four base hits in six innings.

BARCS Animal Shelter in Baltimore City brought their adorable pups, including "Analu" and "Liko" to play on the field with some of the Baltimore Orioles.

The Supreme Court will consider Monday whether bans on public camping constitute "cruel and unusual punishment" barred by the Eighth Amendment.

Hogan hopes to become the first Republican in more than 40 years to win a Senate seat in deep-blue Maryland, where Democrats outnumber Republicans 2-1 statewide.

The Supreme Court seemed divided over a case challenging the scope of a federal obstruction law that prosecutors have used to charge more than 300 Jan. 6 defendants.

The investigation is being conducted by Maryland's U.S. Attorney's Office and the FBI.

The White House is turning to other methods to erase student debt after the Supreme Court blocked its broader effort to forgive loans.

When it comes to vintage style, art and innovation—Baltimore is home to it all.

The University of Maryland Medical Center was granted the American Nurses Credentialing Center's (ANCC) Magnet for the fourth time in a row.

Magali Uroza comes from a Mexican immigrant family and remembers the challenges of learning English while growing up in Baltimore.

It is the cherry trees' second-earliest peak bloom on record and follows one of Washington's warmest recorded winters. In 1990, the trees blossomed on March 15.
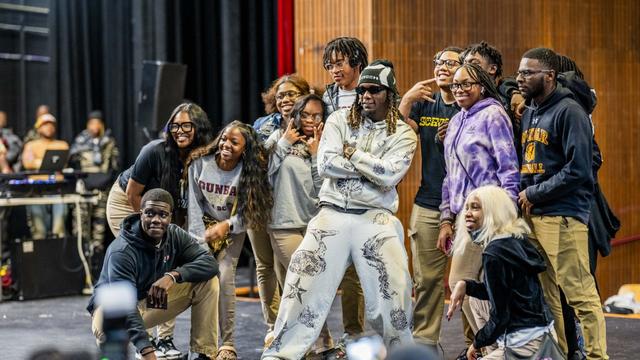
Georgia rapper Offset surprised students at Dunbar High School on Tuesday for their stellar academic performance and attendance according to the Baltimore Police Department.

The Maryland Food Bank is on pace to distribute more than 52 million pounds of food, which is a 20 percent jump in comparison to pre-COVID times.

It has long been a popular resource in the community, easily accessible to many college students and neighbors.

Has mom and dad's bank been open too long at your house?

According to a report from the National Institute on Retirement Security, 40% of Gen Xers have zero dollars saved for retirement.

According to Bankrate, Baltimore is one of the top 10 most affordable cities for buying used cars.

Primanti Bros. on Tuesday announced plans to expand to the Baltimore region this spring.

CVS is closing dozens of pharmacies inside Target stores in 2024 as the store and other retail pharmacy chains face increasing difficulties.

The wildly popular chicken finger joint opened its doors Tuesday at the Snowden River Shopping Center.

The council passed the "Bring Your Own Bag Act" with a bipartisan vote in February.

Baltimore is one of 31 designees announced Monday, picked from nearly 400 applicants.

Most worrisome gaps involve cancer chemotherapy drugs, ER medications and and therapies for ADHD.

The prepackaged boxes of deli meat, cheese and crackers are not a healthy choice for kids, advocacy group says.

The University of Maryland Medical Center was granted the American Nurses Credentialing Center's (ANCC) Magnet for the fourth time in a row.

The state aims to force the company to make necessary repairs and impose fines of up to $25,000 per violation.
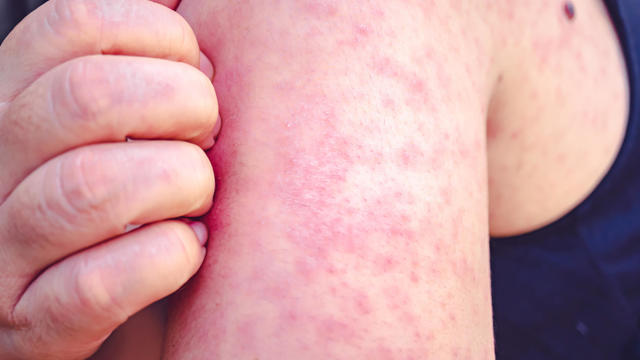
Infections of the highly contagious measles virus have been reported in 17 states in the first three months of 2024.

Charli XCX and Troye Sivan are stopping in Baltimore this fall on a joint tour.

In the 1,000th episode, titled "A Thousand Yards," NCIS comes under attack by a mysterious enemy from the past.

A Billy Joel special on CBS and Paramount+ will air again after it was cut off in the middle of the singer's performance of "Piano Man."

The show, renowned for its thrilling investigations and dedicated team, has become a cornerstone of television drama.

O.J. Simpson was famously acquitted in the 1994 slayings of his ex-wife, Nicole Brown Simpson, and her friend Ronald Goldman.

Rain chances to close out the week later Friday...followed by a dry weekend.

Derek Beasley has your Thursday evening forecast (4/18/2024)
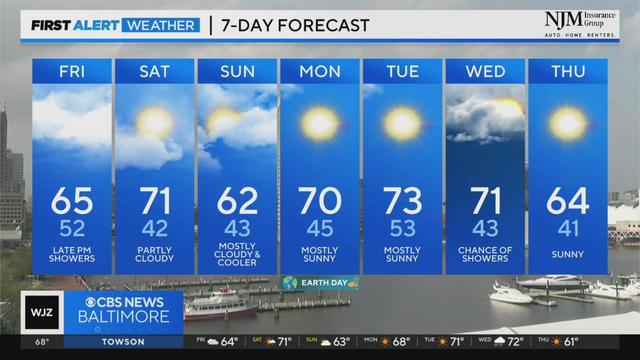
Derek Beasley has your Thursday evening forecast (4/18/2024)

Meteorologist Abigail Degler has your Thursday morning forecast 4/18/2024

Derek Beasley has your Wednesday night forecast (4/17/2024)

Brigance Brigade Foundation set to host annual run to raise money for ALS

Army Corps gives close-up view of Key Bridge collapse site, plus more top news

Derek Beasley has your Thursday evening forecast (4/18/2024)

WJZ, Baltimore Banner, WYPR Radio will host Democratic mayoral debate on April 30

Prigel Family Farm works to expand agricultural education; offers summer camp for children