CBS News Live
CBS News Baltimore: Local News, Weather & More
Watch CBS News

Robinson was one of the three men found in Mexican town during a suffering trip, according to the FBI.

More periods of rain are likely tonight through the day on Sunday. Steady rain tapers off before Sunday evening.

Salvage crews are working Friday to remove a massive piece of the collapsed Francis Scott Key Bridge off the bow of the Dali, the malfunctioning cargo ship that crashed into the bridge in March and is now pinned under tons of steel.

The pro-Palestine protest at Johns Hopkins University entered its fifth day on Friday, with tensions mounting as the encampment continues to grow.

The 25th annual Maryland Film Festival is underway.

One of Baltimore's longest traditions is back: Flower Mart kicked off in Mount Vernon Friday morning.

Eight Maryland first responders, killed in the line of duty, were honored Friday afternoon at the annual Fallen Heroes Day ceremony.

A civil rights complaint was filed by a group of Reservoir Hill residents to the U.S. Department of Transportation that alleges Amtrak's proposed Frederick Douglass Tunnel Program will disproportionately impact Baltimore's Black and low-income community members.

New speed cameras will go into place Monday, according to the Baltimore Department of Transportation.

Cooler temperatures heading into the weekend, temperatures in the low 80's to start the work week.

Maryland Transportation officials say the cost of a new Francis Scott Key Bridge would cost between $1.7 billion and $1.9 billion, and it wouldn't be completed until the fall of 2028.

The body of a fifth missing construction worker was recovered Wednesday from the wreckage site of the Key Bridge collapse.

The policy will only cover a small fraction of the billions in damages and the clean-up costs associated with Francis Scott Key Bridge collapse caused by a cargo ship, the Dali, in Baltimore.

It's official: OBJ is coming to South Florida.

The announcement marks the first time that the Orioles claimed AL Player and Rookie of the Month in the same month.

The Orioles (20-11) have a one-game lead in the American League East after taking three of four from their New York rivals.

The Baltimore Orioles put right-hander Grayson Rodriguez on the 15-day injured list Wednesday because of shoulder inflammation, and they activated lefty John Means.

The Baltimore Orioles were on the short end of a pitchers' duel among the top two teams in the American League East.

Michelle Paris' second book is coming out in May and is called Eat Dessert First—it’s a sweet romance about a plus-sized baker's journey to find the recipe for happiness and love.
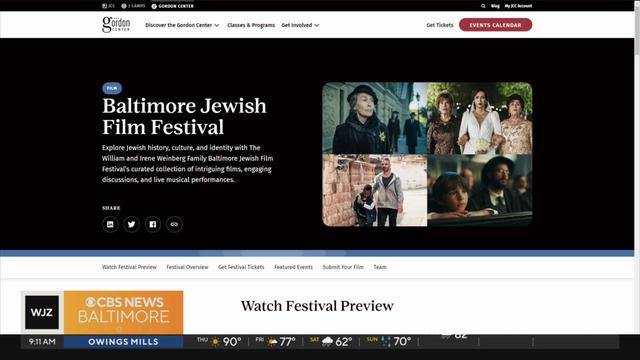
The 36th annual festival presents four engaging films that examine Jewish history, heritage, and culture.

We are live at Sterling Forever this morning to see what they thinnk is the perfect gift for Mom this year.

We are joined in studio by DeAndra Bailey from Baltimore, who is headed to Paris to compete in the Food Network competition.
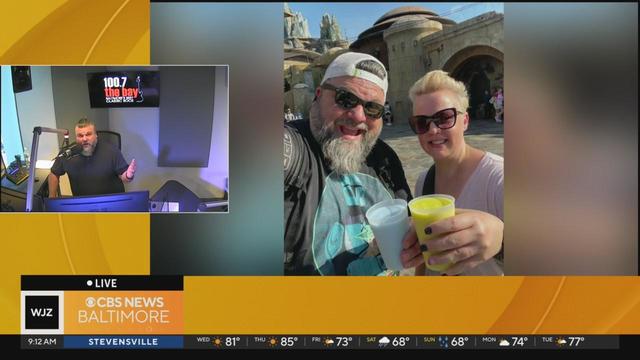
In the debut of "Hangin' with Huber," Huber reflects on a recent family trip to Disney World

The Pratt Library Book Club is kicking off with recommendations from Meghan McCorkell for both adults and kids! Also, don't forget the library's Summer Break Baltimore Program is coming up

Held annually over the past 25 years, the festival has evolved, embracing new technologies, formats, and voices while remaining true to its core mission of celebrating the power of cinema to inspire, provoke, and unite audiences.

Last year Harford Community College hosted the men’s championship for the first time. The NJCAA was so impressed by how Harford handled the championship that it awarded the men’s championship AND women’s invitational to the College through 2028.

The Maryland Department of Agriculture and Hillen Homestead Farm have tips on how to build the best Mother's Day bouquet.

Cooler temperatures heading into the weekend, temperatures in the low 80's to start the work week.

The steel must be removed safety in order for the ship to float again in the Patapsco River.

A backdoor cold front (a front that moves in the opposite direction from what's usually seen) moved through the region last night into this morning.

A civil rights complaint was filed by a group of Reservoir Hill residents to the U.S. Department of Transportation that alleges Amtrak's proposed Frederick Douglass Tunnel Program will disproportionately impact Baltimore's Black and low-income community members.

The DEA is proposing to reclassify cannabis as a less dangerous drug. Christina Johnson of Standard Wellness is talking about what that could mean

Robinson was one of the three men found in Mexican town during a suffering trip, according to the FBI.

More periods of rain are likely tonight through the day on Sunday. Steady rain tapers off before Sunday evening.

Salvage crews are working Friday to remove a massive piece of the collapsed Francis Scott Key Bridge off the bow of the Dali, the malfunctioning cargo ship that crashed into the bridge in March and is now pinned under tons of steel.

The pro-Palestine protest at Johns Hopkins University entered its fifth day on Friday, with tensions mounting as the encampment continues to grow.

The 25th annual Maryland Film Festival is underway.

Salvage crews are working Friday to remove a massive piece of the collapsed Francis Scott Key Bridge off the bow of the Dali, the malfunctioning cargo ship that crashed into the bridge in March and is now pinned under tons of steel.

Federal prosecutors have asked to seize former Baltimore State's Attorney Marilyn Mosby's Florida condo, according to new court filings.

Johns Hopkins University President Ron Daniels is calling on protesters to end their encampment on the campus.

The policy will only cover a small fraction of the billions in damages and the clean-up costs associated with Francis Scott Key Bridge collapse caused by a cargo ship, the Dali, in Baltimore.

To cast your ballots early in Maryland, find an early voting center in the county where you live.

Michelle Paris' second book is coming out in May and is called Eat Dessert First—it’s a sweet romance about a plus-sized baker's journey to find the recipe for happiness and love.

The 36th annual festival presents four engaging films that examine Jewish history, heritage, and culture.

We are live at Sterling Forever this morning to see what they thinnk is the perfect gift for Mom this year.

We are joined in studio by DeAndra Bailey from Baltimore, who is headed to Paris to compete in the Food Network competition.

In the debut of "Hangin' with Huber," Huber reflects on a recent family trip to Disney World

It's official: OBJ is coming to South Florida.

The announcement marks the first time that the Orioles claimed AL Player and Rookie of the Month in the same month.

The Orioles (20-11) have a one-game lead in the American League East after taking three of four from their New York rivals.

The Baltimore Orioles put right-hander Grayson Rodriguez on the 15-day injured list Wednesday because of shoulder inflammation, and they activated lefty John Means.

The Baltimore Orioles were on the short end of a pitchers' duel among the top two teams in the American League East.
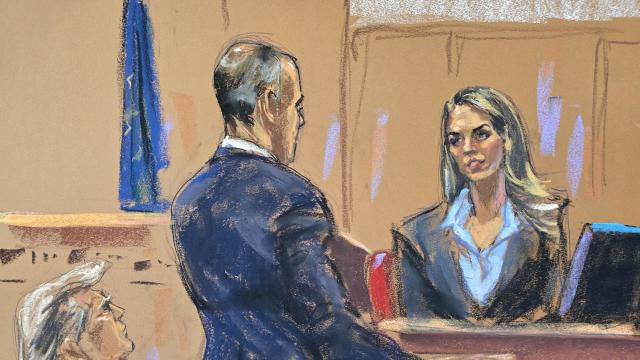
Hope Hicks, one of former President Donald Trump's closest aides for years, told jurors how she handled the fallout from "hush money" payments made to two women before the 2016 election.

President Biden awarded the Presidential Medal of Freedom, the nation's highest civilian honor, to 19 recipients.

To cast your ballots early in Maryland, find an early voting center in the county where you live.

Prosecutors in former President Donald Trump's criminal trial in New York called two new witnesses to the stand on Friday, rounding out the first week of testimony.

Former National Enquirer boss David Pecker appeared on the stand for the third day, detailing an agreement the tabloid made with a former Playboy model.

The theme of the race is "Monuments and Masterpieces." As usual, some parking restrictions and lane closures will be in place Saturday to accommodate the whacky race.

When it comes to vintage style, art and innovation—Baltimore is home to it all.

The University of Maryland Medical Center was granted the American Nurses Credentialing Center's (ANCC) Magnet for the fourth time in a row.

Magali Uroza comes from a Mexican immigrant family and remembers the challenges of learning English while growing up in Baltimore.

It is the cherry trees' second-earliest peak bloom on record and follows one of Washington's warmest recorded winters. In 1990, the trees blossomed on March 15.

The Maryland Food Bank is on pace to distribute more than 52 million pounds of food, which is a 20 percent jump in comparison to pre-COVID times.

It has long been a popular resource in the community, easily accessible to many college students and neighbors.

Has mom and dad's bank been open too long at your house?

According to a report from the National Institute on Retirement Security, 40% of Gen Xers have zero dollars saved for retirement.

According to Bankrate, Baltimore is one of the top 10 most affordable cities for buying used cars.

Primanti Bros. on Tuesday announced plans to expand to the Baltimore region this spring.

CVS is closing dozens of pharmacies inside Target stores in 2024 as the store and other retail pharmacy chains face increasing difficulties.

The wildly popular chicken finger joint opened its doors Tuesday at the Snowden River Shopping Center.

The council passed the "Bring Your Own Bag Act" with a bipartisan vote in February.

Baltimore is one of 31 designees announced Monday, picked from nearly 400 applicants.
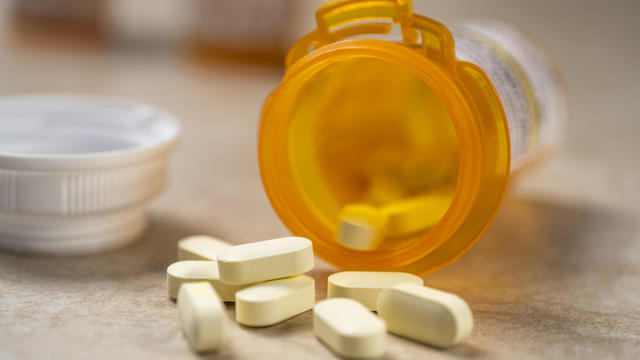
Opioid addictions often start by having access to prescription medications.
Regular screenings are critical to identifying and treating cancer early. It's also important to be aware of any genetic mutations that already exist in your body.

Health officials are warning consumers not to consume Infinite Herbs basil sold at some Trader Joe's and Dierberg's stores after 12 people were sickened.

Most worrisome gaps involve cancer chemotherapy drugs, ER medications and and therapies for ADHD.

The prepackaged boxes of deli meat, cheese and crackers are not a healthy choice for kids, advocacy group says.

The theme of the race is "Monuments and Masterpieces." As usual, some parking restrictions and lane closures will be in place Saturday to accommodate the whacky race.

The nine-time Grammy Award winner performs at CFG Bank Arena on Friday, October 4. It's her fourth stop on an 81-date world tour.

Rapper Busta Rhymes and funk rockers Morris Day and The Time are headlining the 47th annual AFRAM this year in Baltimore.

The significance of the song was amplified by praise from music legend Paul McCartney, who called the recording "magnificent" and appreciated its reinforcement of the civil rights message he intended when writing "Blackbird."

Charli XCX and Troye Sivan are stopping in Baltimore this fall on a joint tour.

More periods of rain are likely tonight through the day on Sunday. Steady rain tapers off before Sunday evening.

Cooler temperatures heading into the weekend, temperatures in the low 80's to start the work week.

A backdoor cold front (a front that moves in the opposite direction from what's usually seen) moved through the region last night into this morning.
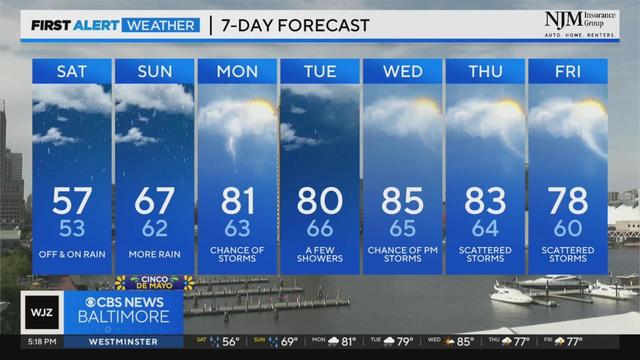
A backdoor cold front (a front that moves in the opposite direction from what's usually seen) moved through the region last night into this morning.
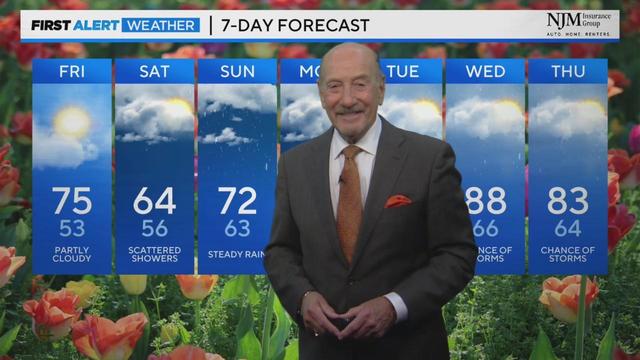
For this morning temps are starting out in the 60s and 70s across the state. We will see partly sunny conditions throughout the next several hours of the morning and into the afternoon.

Cooler temperatures heading into the weekend, temperatures in the low 80's to start the work week.

The steel must be removed safety in order for the ship to float again in the Patapsco River.

A backdoor cold front (a front that moves in the opposite direction from what's usually seen) moved through the region last night into this morning.

A civil rights complaint was filed by a group of Reservoir Hill residents to the U.S. Department of Transportation that alleges Amtrak's proposed Frederick Douglass Tunnel Program will disproportionately impact Baltimore's Black and low-income community members.

The DEA is proposing to reclassify cannabis as a less dangerous drug. Christina Johnson of Standard Wellness is talking about what that could mean